Introduction
The standard regimen with vitamin K antagonist (VKA) and low-molecular-weight heparin (LMWH) was the main therapeutic option in patients with venous thromboembolisms (VTE) until the launch of direct oral anticoagulants (DOAC) in 2013 in South Korea. The ability of health-care provider to make appropriate dosage of VKA (e.g., warfarin) and follow-up decisions can have a major impact on therapeutic effectiveness and safety such as recurrent VTE and bleeding events, respectively [1]. The quality of anticoagulation can be assessed by time in therapeutic range (TTR). A strong relationship between TTR and bleeding or thromboembolic rates has been observed across studies [2]. However, TTR in real clinical practice was lower than 57% to 64% in clinical trials [3]. The objective of this study was to investigate the quality of anticoagulation with TTR in patients with VTE in a single tertiary hospital in Korea.
Methods
Study subjects
This was a retrospective, single-center study to evaluate the adequacy of control for anticoagulation in patients on warfarin to treat VTE. The study population were patients admitted to Soonchunhyang University Hospital in Korea between February 2009 and August 2010.
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of the Soonchunhyang University Hospital (IRB No. SCHUH 2018-07-027-001). Informed consent was waived by the IRB because this was a retrospective study.
Seventy-six patients aged 18 years and over who were receiving warfarin therapy for VTE were investigated. They were managed over two thirds of the predetermined follow-up period at Soonchunhyang Thrombosis Clinic.
Patients were excluded if target range of international normalized ratio (INR) was not 2.0-3.0 according to American College of Chest Physicians (ACCP) recommendation. Patients whose test interval between two consecutive INRs exceeded 56 days were also excluded from statistical analysis. The period of predetermined warfarin interruption for peri-operative management (1 week pre-operatively and 3 weeks post-operatively) was censored for 34 episodes. Thus, they were not analyzed. Out of 76, 47 patients who had available data over at least 2 months were included for final analysis.
Dosing algorithm
If the INR was in therapeutic range, there was no change of dose. A 10% adjustment in the weekly dose of warfarin was used when two consecutive INR results were out of range by no more than 0.5 units below or 1.0 unit above the therapeutic INR range while a 10-20% adjustment in the weekly dose of warfarin was used if the deviation from the therapeutic INR range was more extreme [4].
TTR was the percent of time in which patients with VTE on warfarin had INR results within the therapeutic range (2.0-3.0) during the test period. The TTR was calculated using linear interpolation method of Rosendaal [5].
Outcomes
Primary outcome was TTR. The primary safety outcome was the incidence of major bleeding which was defined as overt bleeding leading to a ≥ 2 g/dL drop in hemoglobin level, transfusion of ≥ 2 units of packed red blood cells, need for re-operation or invasive intervention, any bleeding at a critical anatomic site (e.g., intracranial, retroperitoneal, intraocular, or pericardial), or fatal bleeding [6].
Results
Patients characteristics and procedure
Out of 76 patients between February 2009 and August 2010, 47 (23 men; mean age 64.9 years; range, 27-86) were analyzed in this study. Approximately two-thirds (31/47) of these patients were warfarin-naïve. Expected duration of anticoagulation was 6 months in 22 patients (47%), 12 months in 1 (2%), and indefinite in 24 (51%). There were various comorbidities, including active malignancy in 7 (15%), mycobacterium tuberculosis on rifampicin in 4 (8.5%), chronic kidney disease in 4 (8.5%), and hemorrhagic cerebral infarct in 1 (2%) (Table 1). There was no patient whose interval between two consecutive INRs exceeded 56 days.
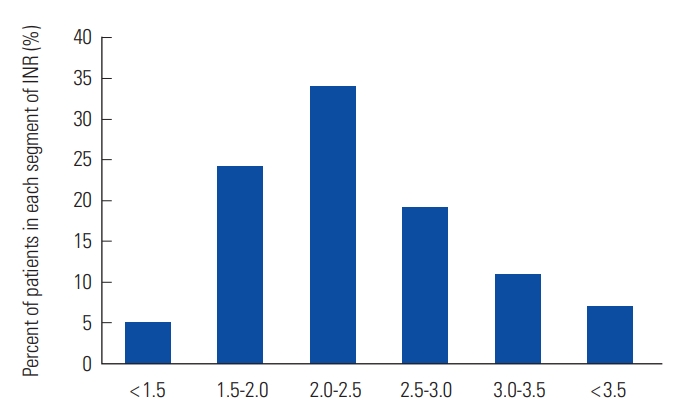
Overall TTR was 52.4%. Proportions of out-of-range below and above the therapeutic range were 29.5% and 18.2%, respectively (Table 2). Fig. 1 shows the proportion of patients corresponding to subdivided segment of INR value. The period of predetermined warfarin interruption for peri-operative management (1 week preoperatively and 3 weeks post-operatively) was censored for 34 episodes that were not analyzed. Total number of hospital admission was 84. There was no thromboembolic complication, although two cases (4.3%) of major bleeding complications were observed: a 79-year-old female patient had upper gastrointestinal bleeding on the 4th day of warfarin treatment with concomitant aspirin medication; and a 52-year-old male patient had intramuscular bleeding with high INR of 4.16. All-cause mortality was 0% (0 patient) (Table 3).
Discussion
This study shows that validated dosing algorithm is effective and safe in getting INRs within therapeutic range for patient with VTE. In this study, overall TTR was 52.4%. This study showed no thromboembolic complication (0%), although two cases of major bleeding complications (4.3%) were observed. The incidence of recurrent VTE could be between 4.6% to 4.9% based on the study of Veeger et al. [7] who reported annual incidences of recurrent VTE and major bleeding patients with individual TTR (ITTR) of 45% to 65% were similar to those in patient with ITTR of >65% (4.9% vs. 4.6% and 2.1% vs. 1.9%, respectively). Patients with ITTR <45% were at higher risk of recurrent VTE than those with ITTR >65% (RR 2.8, 1.9-4.3, P<0.001).
Walraven et al. [8] have shown that TTR in patients managed in anticoagulation clinic is 65.6% compared with 56.7% in community setting. Manotti et al. [9] showed that TTR in patients managed with computer-aided dosing system was 71.2%. TTR in thrombosis clinic of Soonchunhyang University Seoul Hospital and usual clinical practice were 52.4% and 43.7%, respectively [10]. This study shows that warfarin dosing algorithms are very attractive method to improve the TTR in patients with VTE in Korea [4]. In this study, the bleeding rate (4.3%) was higher than the rate of 2.1% in patient with ITTR of 45% to 65% in the study of Veeger et al. [7] In subgroup analysis of Hokusai-VTE trial, Nakamura et al. [11] have suggested that higher risk of bleeding is expected when INR is maintained at 2.0-3.0 given that Japanese guidelines recommend a target INR range of 1.5-2.5 [12]. A Chinese study recommended a target INR range of 1.8-2.4 [13]. Korean physicians might tend to maintain lower INR level because of bleeding concerns, considering that 29.5% were INR <2.0 but only 18.2% were INR >3.0 as shown in this study. The optimal target level of INR in Korean population needs to be further evaluated in terms of safety.
This study has some limitations that should be addressed. Firstly, this was retrospective study at a single center. Secondly, the population size of this study was too small (n=47) to generalize results of this study to clinical practice. Thirdly, there was no comparable group regarding anticoagulation management.
This is already the era of DOACs. However there are still patients who need to take VKA in the real world [14]. Moreover, we have to go back to warfarin to avoid (1) drug interaction with rifampicin, P glycoprotein, (2) contraindication such as patients with renal failure, or (3) high cost after only 6-month period of insurance in Korea. In this context, this study is very meaningful for patients who have to reach therapeutic INR level in clinical practice.
In conclusion, the validated dosing algorithm is effective for managing INRs in patients with VTE in Korea.