Introduction
Warfarin is the most commonly-used oral anticoagulant for the prevention and treatment of for atrial fibrillation, heart valve disease or replacement, deep vein thrombosis, and pulmonary embolism. Due to the narrow therapeutic index and wide interindividual variability in warfarin dose requirements, it is essential to monitor the international normalized ratio (INR) for patients receiving warfarin. Warfarin is metabolized extensively through the liver and administered as a racemic mixture of the R- and S-stereoisomers. The cytochrome P450 (CYP) enzyme CYP2C9 is responsible for the metabolism of S-warfarin which is 3–5 times more potent than R-warfarin [1]. Another cytochrome P450 enzyme, CYP3A4, metabolizes R-warfarin [2]. For this reason, one of the disadvantages of warfarin is the numerous drug-drug interactions that can occur, as the cytochrome P450 enzyme also metabolizes other drugs (Table 1). When co-administered, these drugs can subsequently affect the INR [3].
Voriconazole is a broad-spectrum antifungal triazole recommended as the primary therapy for invasive aspergillosis [4]. Voriconazole is a substrate for CYP2C9, CYP2C19, and CYP3A4. It is also a strong inhibitor of CYP3A4 and a moderate inhibitor of CYP2C9 and CYP2C19 (Table 1), and the co-administration of drugs that modulate or affect CYP2C9 or CYP3A4 activity may affect plasma concentrations of voriconazole. Due to such drug-drug interactions, the package insert voriconazole warns of the risk for prolonged bleeding times when prescribing warfarin [5].
Although the potential for such drug-drug interactions are known, there have yet to be reports of real-world cases involving voriconazole and warfarin. We report a case of a patient receiving chronic warfarin therapy in a stable phase. Upon receiving voriconazole, fluctuations in her INR value were observed, and she was successfully managed with close INR monitoring and appropriate warfarin dose adjustments.
Case presentation
A 61-year-old female with a past history of atrial fibrillation (AF), moderate mitral stenosis (MS), diabetes mellitus (DM), and asthma was receiving a weekly warfarin dose of 24-28 mg, and the patient’s INR was relatively stable. Her genetic polymorphisms included VKORC1-1639G>A GA, CYP2C9*3 42614A>C AA and CYP4F2*3 18000G>A GG.
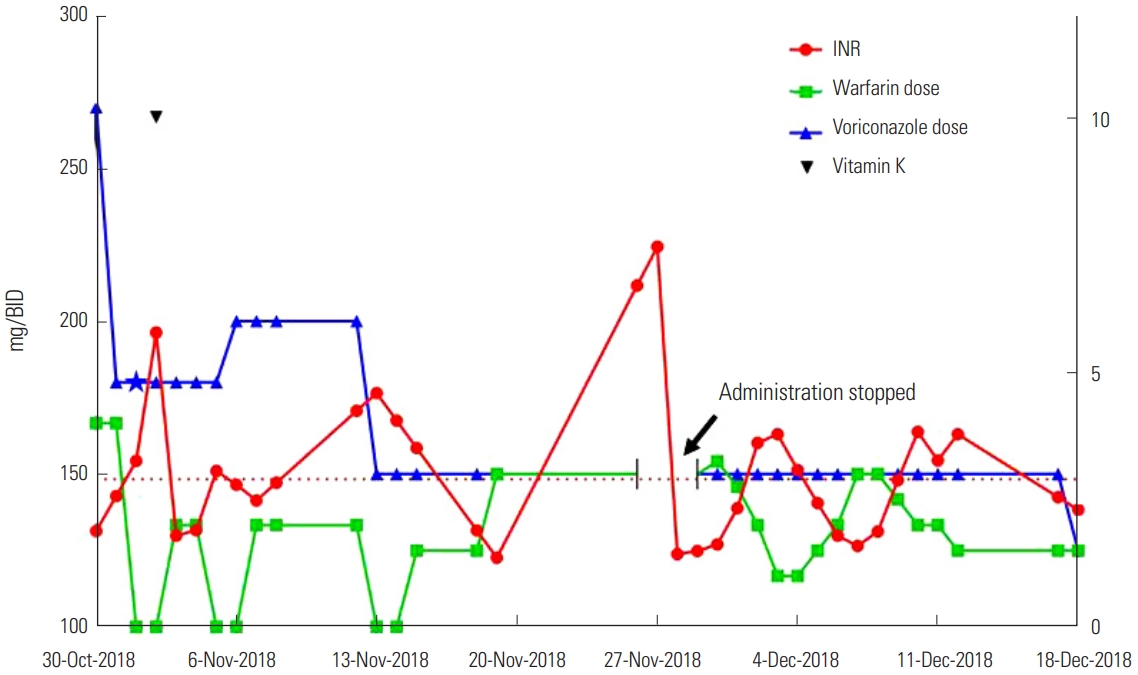
The patient presented with intermittent right flank pain on October 4, accompanied by cough, with the pain worsening after each cough. Chest CT showed multifocal peribronchial consolidation with ground-glass opacity (GGO) in the right lung. After administering oral levofloxacin for one week, the cough persisted. She was then admitted to hospital, where a bronchoscopy test revealed lower lobar bronchial stenosis and she was positive for serum galactomannan. Bronchoalveolar lavage (BAL) gram culture confirmed the presence of Pseudomonas aeruginosa. The results of BAL fluid analysis were turbid with pH: 7.0, WBC: 2,850/mm3, and polymorphic WBC: 75%. A further chest CT showed airway invasive aspergillosis (IA) and she was prescribed voriconazole (Vfend™, Pfizer). Initially, the patient began receiving intravenous voriconazole (270 mg, twice daily) and oral warfarin (4 mg/day), and the INR value was 1.88. The following day, the voriconazole dose was reduced to 180 mg twice daily and the warfarin dose was unchanged. On the third day, the patient was not administered with warfarin owing to a high INR value (3.26). The INR value remained high the following day (5.78), although the patient did not show any symptoms such as bleeding, and was treated with intravenous vitamin K1 at a dosage of 10 mg, with the warfarin treatment still halted. Over the next 2 days, her INR value stabilized and warfarin treatment was restarted (2 mg/day). On the 7th day after beginning the administration of voriconazole, the intravenous voriconazole therapy (180 mg twice daily) was changed to oral treatment (200 mg twice daily) and warfarin was still omitted due to the high INR value (3.06). During this week, the weekly warfarin dose of 12 mg was reduced by more than 50% of the patient’s standard dose owing to the interact with voriconazole. Two days later, the patient was discharged after her symptoms improved. She discharged with voriconazole oral treatment (200 mg, twice daily) and warfarin (2 mg/day), and her INR was 2.48 at that time. In the second week after combining the voriconazole and warfarin treatment, the warfarin dose was 12 mg/week.
In the first outpatient clinic (Nov. 13) after discharge, the doctor recommended adjusting the oral warfarin dose to 1.5 mg daily after skipping warfarin for 2 days as the INR value was 4.59. The voriconazole oral dose was also reduced to 150 mg twice daily because the voriconazole therapeutic drug monitoring (TDM) value was 4.55 μg/mL. In the second outpatient clinic (Nov. 19), the INR value was 1.36. The doctor recommended increasing the oral warfarin dose to 3 mg daily, with the voriconazole oral dose maintained at 150 mg twice daily. In the week of combined use of warfarin and voriconazole, the warfarin weekly dose was 9 mg, representing a 63-68% reduction from her normal warfarin dose owing to the interaction with voriconazole. After November 19, the fourth week of combination treatment, the weekly dose was 21 mg, a reduction of 13-25% from the normal warfarin dose. In the third outpatient review (Nov. 27), the patient presented with easy bruising and mild epistaxis, with an INR value of 7.46. She was treated with an intravenous dose of vitamin K1 10 mg again and warfarin treatment was omitted for 2 days. Voriconazole treatment was also halted, before being changed to cefditoren oral treatment (100 mg, three times a day). She was told by her physicians to return to hospital at anytime if she felt uncomfortable.
On November 28, she presented with a sore throat and general weakness, and was hospitalized again. Owing to the prolongation of PT time, warfarin treatment was halted that day. On the second day after admission (Nov. 29), her INR value was 1.49, and she was treated with oral voriconazole 150 mg twice daily and oral warfarin 3 mg daily. During the first week of hospitalization, the warfarin dose was dynamically adjusted based on the INR value. The highest INR value was 3.78 during this week and the corresponding warfarin dose was 1 mg/day. The weekly warfarin dose was 14.5 mg, a reduction of more than 40-48% from the normal warfarin dose owing to the interaction with voriconazole. During the second week of hospitalization, the highest INR value was 3.83 and the corresponding warfarin dose was 2 mg/day. The weekly warfarin dose was slightly increased to 16 mg. At the end of the 6th week after the beginning of warfarin and voriconazole combination therapy (December 12), she was discharged with a prescription of oral voriconazole 150 mg twice daily and oral warfarin 1.5 mg daily with an INR of 3.78 and was recommended for regular review. During her hospitalization, she did not present with bleeding, cough, fever, or other adverse complaints when receiving combined warfarin and voriconazole. Six days later, on December 18 during the first outpatient follow-up, the patient reported no discomfort or complaints. In accordance with the TDM (5.54 μg/mL), the adjusted voriconazole dose was reduced to 125 mg twice daily. The warfarin dose was 1.5 mg/day and her INR was 2.30. In the 7th week, the weekly warfarin dose was adjusted to 10.5 mg which was 34% lower than the previous week. Eventually she was stabilized on the 10.5 mg/weekly warfarin dose 6 weeks post-voriconazole therapy and her INR value was 2.30 (Figure 1).
Discussion
To our knowledge, this is the first known case report describing the successful management of a fluctuating INR involving the interaction between voriconazole and warfarin. These observations provide insight into the effects of long-term use of voriconazole on INR. In addition, in order to maintain therapeutic INR, warfarin doses should be adjusted by approximately 13-68% during the concurrent use of voriconazole.
This patient had a previous history of atrial fibrillation and MS, and long-term use of warfarin anticoagulant therapy. According to guideline recommendations, the INR target level was maintained between 2-3 for the prevention of stroke in AF patients with moderate-to-severe mitral stenosis, and the patient could be considered to be in the stable anticoagulation stage [6]. Her genetic polymorphisms of VKORC1-1639G>A GA, CYP2C9*3 42614A>C AA, and CYP4F2*3 18000G>A GG meant that she required a higher warfarin of dose in order to have the same anticoagulation effect. The increase in INR occurring during the course of her treatment was presumed to be due to the drug affecting warfarin metabolism resulting in an increase in plasma drug concentrations. The attending physicians avoided using any other drugs that may affect warfarin metabolism with the exception of voriconazole during her treatment.
Invasive aspergillosis (IA) can produce severe fungal infections with high mortality. IA frequently appears in patients undergoing transplants and those receiving chemotherapy and immunosuppressive therapy. Classic at-risk patients for IA include those with prolonged neutropenia, recipients of hematopoietic stem-cell transplants or solid-organ transplantations, and patients with advanced acquired immunodeficiency syndrome or chronic granulomatous disease or preexisting structural lung disease. The presence of a coexisting condition such as diabetes or malnutrition is one of the risk factors. Fever, cough with or without sputum production, and dyspnea are frequent, although nonspecific clinical manifestations of pulmonary aspergillosis and presence in the lung is common. The diagnosis of IA remains difficult due to the non-specific symptoms. The earliest radiologic sign of IA via chest computed tomography (CT) is the appearance of a nodule. A “halo sign” can be suggestive of IA in patients with compatible host factors. Cultures of bronchoalveolar lavage fluid (BAL), the galactomannan assay or aspergillus polymerase chain reaction (PCR) assay are relatively sensitive and specific for the diagnosis of IA. In a patient who is at high risk of IA and who has a compatible chest CT lesion (e.g., nodule or infiltrate), a positive galactomannan assay or culture of an Aspergillus species from respiratory secretions strongly supports the IA diagnosis. At present, voriconazole is considered the primary therapy for IA [4].
Our patient had a history of diabetes, a risk factor for IA, and chest CT indicated multifocal peribronchial consolidation with GGO in the right lung and airway IA at different times. The serum galactomannan assay was positive and BAL gram culture assay indicated the presence of Pseudomonas aeruginosa. With a clear diagnosis of invasive aspergillosis, she was treated with voriconazole.
Voriconazole is a broad-spectrum triazole antifungal agent that inhibits ergosterol synthesis by inhibiting lanosterol cytochrome P450 14-α-demethylase. It is recommended as primary therapy for IA and as an alternative therapy for candidemia. Oral bioavailability is approximately 95% when doses are administered 1 hour prior to or after a meal. Voriconazole has linear elimination characteristics in children but exhibits nonlinear elimination in adults, which can lead to different dosing selections being required. The linear pharmacokinetics in children results in the clearance of voriconazole more rapidly than in adults and necessitates a higher dose to achieve similar exposure to adults. Voriconazole for the treatment of IA in adults is typically administered as an initial intravenous loading dose of 6 mg/kg for 2 doses, 12 hours apart, followed by 4 mg/kg every 12 hours for maintenance. The recommended oral therapeutic dose in adults is 200–300 mg twice daily or 3–4 mg/kg twice daily. A common adverse event of voriconazole is reversible visual disturbances, with other known side effects including transaminase elevation and skin rash. Due to the pharmacokinetic variability of voriconazole and to avoid the adverse events associated with elevated concentrations and inefficacy at low concentrations, therapeutic drug monitoring (TDM) is recommended. A voriconazole serum concentration of 1.0–4.0 μg/mL has been suggested to treat IA. In the present case report, the voriconazole dose was adjusted by TDM to ensure correct administration. Voriconazole is hepatically metabolized by the CYP450 system and is an inhibitor and a substrate for CYP2C9, CYP2C19, and CYP3A4 [4,5,7,8]. These isoenzymes account for the majority of drug-drug interactions associated with voriconazole. CYP2C9 and CYP3A4 also play roles in the conversion of warfarin to its active metabolite. Due to the increase in concentration by voriconazole, INR monitoring and dose reductions are essential when coadministered with warfarin [9].
The potential for voriconazole to interfere with warfarin’s anticoagulant efficacy was first identified by Puekins et al., [10] via the prolongation of prothrombin (PT) time. In this double-blind, placebocontrolled, two-way crossover study in healthy males with coadministration of voriconazole and warfarin, the mean area under the effect curve (AUEC) value in the voriconazole administration period was significantly greater (3211 s.h) than in the placebo period (2282 s.h) [95% confidence interval (CI) 574, 1,283; P=0.0002]. The mean maximum change from baseline PT time was approximately 2-fold greater between the voriconazole (17 sec) and placebo (8 sec) treatment periods (95% CI 5, 12; P=0.0004). Furthermore, at 144 hours post-warfarin dosing, the PT time was still increased by a mean of 5.4 seconds in the voriconazole plus warfarin-treated group compared with a mean of 0.6 seconds in the placebo plus warfarin-treated group.
In a retrospective study with 29 Japanese patients administered with warfarin and fluconazole, voriconazole, or itraconazole, voriconazole augmented the anticoagulant activity of warfarin and prolonged INR [11]. Mean INR values in 5 of the patients taking voriconazole significantly increased from 1.95 to 2.89 (P<0.05). The INR in 2 out of the 5 patients receiving voriconazole increased by 172% above that observed with warfarin alone on day 2 and to 167% on day 3, representing an approximate 1.7-fold increase in INR. As a consequence, one patient was discontinued for warfarin treatment. The warfarin sensitivity index (WSI) was used to evaluate the effect of azoles and calculated as the INR/daily warfarin dose. WSI increased from 1.13 to 2.23 with voriconazole, which was not statistically significant, although the limited number of patients receiving voriconazole in this study should be considered. The researchers also assessed the relationship with INR and WSI during warfarin treatment and during coadministration of azoles. INRs observed during azole coadministration (Y) correlated significantly with the values observed in the absence of azoles (X) (voriconazole, Y=2.13X−1.27, r2=0.93). WSI for warfarin alone and warfarin plus VRCZ also showed a significant correlation (voriconazole, Y=2.90X−1.04, r2=0.99). It is therefore recommended to regularly monitor INR and adjust the warfarin dose when voriconazole is administered with warfarin.
Conclusion
In this case study, the warfarin concentration accumulated and the INR value increased likely due to voriconazole interactions. In such circumstances, close monitoring of INR is required to avoid adverse events. In order to reduce fluctuations in warfarin plasma concentrations, the avoidance of voriconazole should be considered when patients are also receiving warfarin. Where this is unavoidable, attention should be paid to dynamic dose adjustment and daily monitoring of the INR value so that warfarin dosing occurs safely and effectively.